
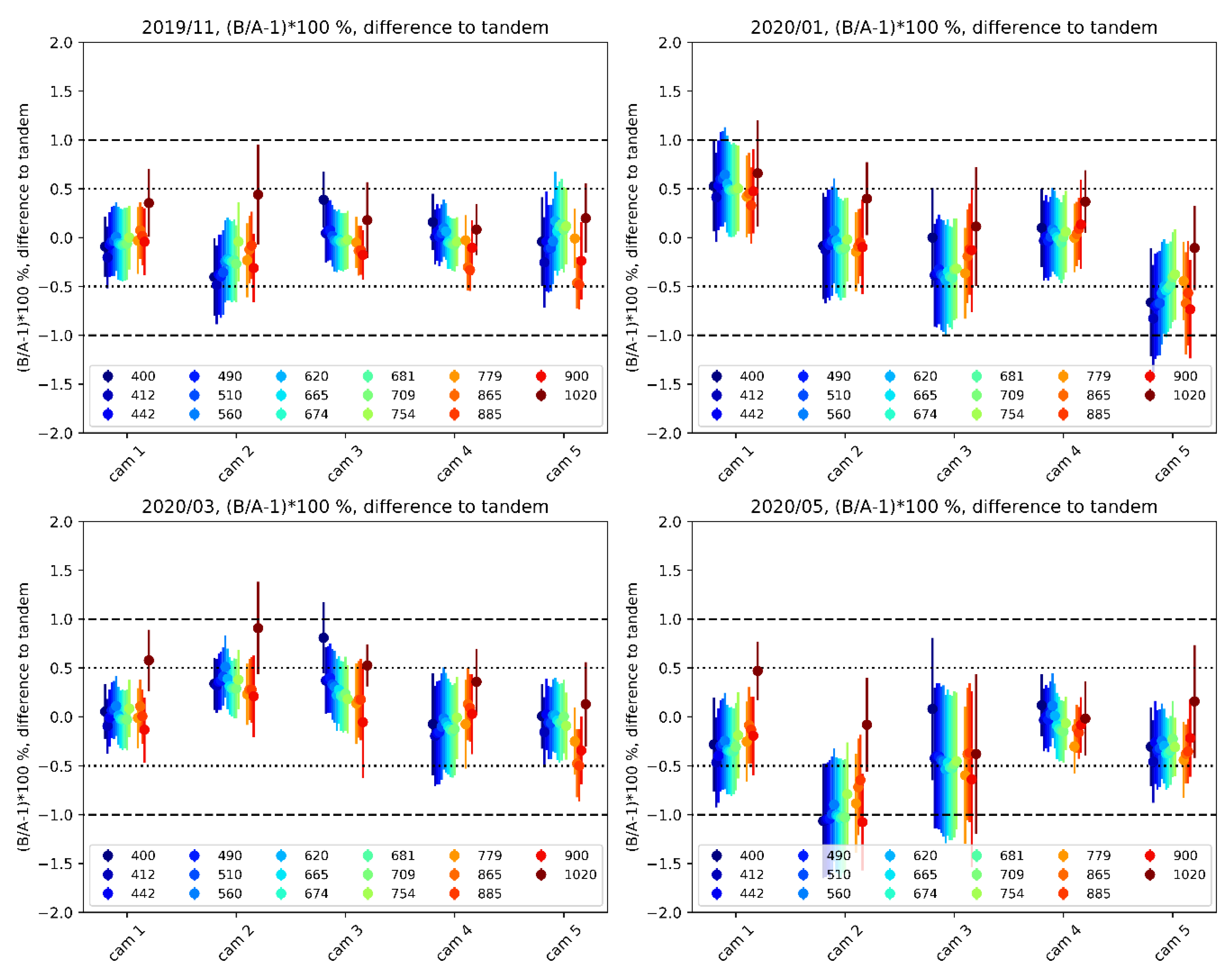
(1−5) Indocarbocyanine dyes, such as Cyanine3 (C圓), Cyanine5 (Cy5), Cyanine7 (Cy7), and Alexa Fluor 647 (AF647), are popular choices for fluorescence-based applications due to their high molar extinction coefficients and tunability of fluorescence wavelengths as well as commercial availability, including their derivatives designed for target-specific labeling. We demonstrate that the potentially deleterious photoconversion, however, can be exploited to develop a new photoactivation method for high-density single-particle tracking in a living cell without using UV illumination and cell-toxic additives.Ĭyanine dyes are indispensable fluorophores for modern chemical and biological studies and have been extensively adopted in single-molecule studies and super-resolution imaging. The formation of a blueshifted congener dye can obscure the multicolor fluorescence imaging, leading to misinterpretation of the data. We also show that the deletion of a two-methine unit from the polymethine chain, which results in the formation of blueshifted products, commonly occurs in other cyanine dyes, such as Alexa Fluor 647 (AF647) and Cyanine5.5. The carbonyl products generated from singlet oxygen-mediated photooxidation of Cy5 undergo a sequence of carbon–carbon bond-breaking and -forming events to bring about the novel dye-to-dye transformation. Our studies show that the formal C 2H 2 excision from Cy5 occurs mainly through an intermolecular pathway involving a combination of bond cleavage and reconstitution while unambiguously confirming the identity of the fluorescent photoproduct of Cy5 to be C圓 using various spectroscopic tools. Here, we report the mechanism for the photoconversion of Cy5 to C圓 that occurs upon photoexcitation during fluorescent imaging.

However, recent observations that blueshifted derivatives of Cy dyes are formed via photoconversion have raised concerns as to the potential artifacts in multicolor imaging. Cyanine (Cy) dyes are among the most useful organic fluorophores that have found a wide range of applications in single-molecule and super-resolution imaging as well as in other biophysical studies.


 0 kommentar(er)
0 kommentar(er)
